Author: Eual A. Phillips
School/Organization:
Hill-Freedman World Academy
Year: 2016
Seminar: The Evolving Universe
Grade Level: 10
Keywords: algebra, atoms, Chemistry, fission, fusion linear equations, graphing, High School, Medicine, modelling, neutrons, nuclear, nuclides, Physical Science, physics, protons, radioactivity, reactions, Science, stars, stellar nucleosynthesis, universe
School Subject(s): Chemistry, Science
Teaching students how the invisible world can be very challenging when to date, no single person knows what the atom physically looks like. The key to getting students to understand atomic structure is to provide them with a variety of learning opportunities that adds depth to their understanding. This curriculum unit encourages students to take on the identities of atoms and to understand how their identity is deeply rooted in the nucleus. Students will embark on a journey of discovering how atoms are created through stellar nucleosynthesis and supernovae explosions by creating physical and mathematical models to discover processes such as alpha process, alpha decay, and beta decay. The goal is for students to realize how studying the origins of the elements inspires scientists replicate those stellar processes in order to make technological and medical advancements. This curriculum unit is written for high school chemistry students in grade 10 who are studying chemistry. This unit incorporates rigorous scientific practices aligned with the International Baccalaureate Middle Years Program.
Download Unit: 16.02.07-unit.pdf
Did you try this unit in your classroom? Give us your feedback here.
Using the standards listed by the School District of Philadelphia, I have found that trying to teach the entire structure of the atom can be a daunting amount of information for the students. In the past, I have broken atomic structure down into two units. The first unit focuses on the nucleus, featuring the protons and neutrons, while the second unit is completely devoted to electron configuration. Despite splitting the units, I have discovered that students will still confuse the roles of the subatomic particles. The current curriculum provides students with an introduction to the nucleus and a heavy focus on the electrons, minimizing the importance of the nucleus. The nucleus is then reintroduced in a nuclear chemistry unit near the end of the school year. An entire academic year is a large enough time gap for students to completely forget how to identify the nucleus of atoms. Outside of counting protons and neutrons in the atomic structure unit, the nucleus is merely a fading vapor in its lifetime; it becomes meaningless and forgotten. To respond to this shallow teaching approach of the atomic structure, I will take a bolder approach and support the unit on the nucleus with the introductory nuclear chemistry recommended by the Next Generation Science Standards (NGSS). The initial extent of the nucleus stops at nuclear notation, which quickly becomes repetitive and mundane. The antidote for the mundane is to provide meaningful variety that accomplishes the same objective. Thus, I believe that an introduction to nuclear chemistry is the appropriate vehicle increase variety. There are four nuclear processes that NGSS recommends students learn in chemistry and physics: alpha process, alpha decay, beta decay, and gamma decay. There are two methods that will allow students to see the importance of the nucleus. The first goal is to connect their souls to the content by relating personal identity with atomic identity. The creative process of the elements will be explored beginning with stellar nucleosynthesis. Ultimately, students will be introduced to the life applications of nuclear chemistry processes observed in stars so that they may see chemistry’s importance in their daily lives.
Student Audience: The unit is intended for students in grade 10. They will attend classes together as an advisory. Students have 60-minute class periods three days per week and 50-minute class periods twice weekly. An estimated 85% of the students will have only completed algebra I, remaining students will have completed algebra II. The students who have completed algebra II are in the same advisory. Grade 10 students are also committed to completion of a personal project as a requirement for finishing the International Baccalaureate Middle Years Program. Grade 10 students will also be in year two (2) of a science, technology, engineering, and mathematics course. Key and Related Concepts: Key concepts are used to paint a broad picture of learning expectations that can be observed in multiple disciplines. They promote both intra-disciplinary and interdisciplinary learning. There are three key concepts that IB recommends as the most prominent in science: change, relationships, and systems. This unit will focus on the key concepts of change and systems, while occasionally referring to relationships as necessary. Related concepts are more discipline-specific and allow for exploration of the key concepts in greater detail in order to cultivate students’ conceptual understanding. The two related concepts selected for this unit’s presentation are form and identity. Other related concepts become applicable throughout the unit. For example, creating models is an important part of the scientific process, but may not necessarily be evident in each lesson throughout the unit. Thus, other related concepts in this unit are interactions, models, and patterns. Conceptual Phrase: The conceptual phrase is a generic statement that summarizes a concept, is nonspecific, and can be applied across multiple content areas and topics. The conceptual phrase developed for this unit is as follows: systemic changes can influence form and identity. This conceptual phrase can be translated to other topics, such as ion formation or the prediction chemical reactions. An additional conceptual phrase for specific lessons in this unit is as follows: modeling relationships and interactions can reveal patterns. This conceptual phrase can also be used translated to topics such as periodicity and reaction rates. Thus, students can benefit from learning conceptual phrases because they potentially enable students to access prior knowledge and to discover similarities and differences between topics in chemistry. Global Contexts: The global context describes the direction of the content and how students will purposefully and meaningfully engage the content. For this unit, the global context will focus on scientific and technical innovation, by which students will explore the natural world and its laws and how humans use their understanding of scientific principles. Students will begin the unit by learning about the basic structure of the atom, focusing specifically on the nucleus. Once students demonstrate mastery in counting protons, neutrons, electrons, and atomic mass, they will be exposed to origin and creation of elements, primarily through stellar nucleosynthesis. Then, like current scientists, they will learn how to predict the formation of elements and understand how those creative processes have application in the medical field. While the primary global context is appropriate, a secondary global context of identities and relationships will be used draw students closer to the content on a personal level. Inquiry Statement: The inquiry statement is comprised of the key and related concepts, the conceptual phrase, and the primary global context. It is provided to the students so that they may know the direction of the unit. The inquiry statement addresses what the student should expect to learn and why they should learn the forthcoming content. The inquiry statement derived for this unit is as follows: Studying systemic changes and their influence on form and identity have helped humans understand the universe in order to modernize medicine. Inquiry Questions: Inquiry questions are provided for students as a guide to what they should learn. They are not necessarily exam questions. The inquiry questions almost serve as an overview or study guide for the entire unit and provoke students into learning the content. Below are the several examples of inquiry questions that can be used throughout the unit. Factual Questions: Conceptual Questions: Debatable Questions: The Objectives of the unit will include the following:
This unit will use a variety of strategies to add depth to students’ understanding of the nucleus of atoms. Using the scope and sequence listed by the School District of Philadelphia, I have found that trying to teach the entire structure of the atom can be a daunting amount of information for the students. Therefore, the atomic structure unit will be broken into two units. The first unit on atomic structure will focus on atomic theory and an introduction to nuclear chemistry. There will be opportunities for students to reflect on their own lives, inquire of the creation of the elements, model nuclides physically and mathematically, and explore the applications of nuclear chemistry. At the conclusion of this unit, then the second unit on atomic structure will feature the electrons, setting the pace for the rest of the chemistry course. Assessment Objectives: The International Baccalaureate (IB) Middle Years Program (MYP) requires that students are assessed according to four criteria. These criterions are defined as (a) knowing and understanding, (b) inquiring and designing, (c) processing and evaluating, and (d) reflecting on the impacts of science. Activities presented in this curriculum unit will primarily assess strands in criterions a, c, and d. Approaches to Learning
Three lessons have been designed in order to enhance student learning in the classroom. The first lesson focuses on engaging the student and using what the student knows about his personal identity in order to gain insight into the identity of atoms. The second lesson uses nuclear notation to write nuclear equations that tell the story of stellar nucleation. The third lesson utilizes a graphical approach to show students that nuclear composition gradually changes as elements become heavier, indicating that other processes are necessary to produce heavier elements. Supplemental materials and ideas are provided at the conclusion of the third lesson. Lesson: Discover Your Atomic Identity Purpose: The purpose of this lesson is for students to understand how the subatomic particles influence the identity of atoms. This lesson will require two days of classroom time. Students will examine their own identities first. Then they will learn about the identity of atoms using prompts, lists, and an expert jigsaw activity. Instructional Objectives: At the end of this lesson, students will be able to describe the evidence for the existence of electrons, protons, and neutrons in order to describe the properties of these subatomic particles. Materials and Equipment: Chart paper, textbooks, computer with internet access, and markers. Engage: Students will be prompted to create 3 lists with each list having 3 activities or actions that they personally perform. The three prompts are each related to the three subatomic particles and their function. The students may remember the subatomic particles from middle school, but may not necessarily understand their function. Therefore, this activity focuses heavily on using their reflections as gateways to understanding the functions of the subatomic particles. Begin the lesson by asking the students to Think-Pair-Share-Write after each prompt below is presented. Subatomic Particles Jigsaw and Gallery Walk: Students will engage in a subatomic particles Jigsaw. Using the textbook and online resources, students will get into 8 groups. For large classes, each topic for the jigsaw will be assigned twice. Each group conduct research on a specific subatomic particle. Afterwards, students will go back to their home groups teach the information to their peers. The groups are named as follows: Rules to the Jigsaw and Gallery Walk Students should be assessed to determine how well they educated one another with your guidance. The subsequent lesson(s) should include quick lab experiments that verify the existence of the subatomic particles and instruction in regards to calculating the number of subatomic particles in each unique atom or isotope. Lesson: Nuclear Notation Purpose: The purpose of this lesson is for students to learn how scientists communicate atoms through literature on an international level. Students will learn nuclear notation and will be able to identify individual isotopes based on their mass and atomic numbers. This lesson should be taught after students have learned how to calculate the number of subatomic particles in an atom given the atomic number and/or the mass number. Materials & Equipment: PowerPoint slides, smart board, projector, colored chalk or white board pens, printed diagrams, isotope puzzle cubes made using origami.[1] Do Now Activity: Isotope Puzzle Cubes Group Activity: Divide the class into groups of six and supply each group with a cube that a nuclear symbol written on each face. Each nuclear symbol should be missing one of the three parts of the symbol, such as in the following examples: Lesson: Stellar Nucleosynthesis Instructional Objectives: At the end of this lesson, the students will be able to define alpha process and discuss nuclides in terms of their number of subatomic particles in order to explain how elements are created naturally, i.e. nucleosynthesis, and artificially. Engage: In order to create interest and generate curiosity, students will watch The Science of the Avengers Video[2], and journal their response to an instructional prompt as their kick-off activity, beginning at time 1:00 and ending at 1:40. The strength of this video lies in its reference to Tony Stark and his ability to synthesize a new element when he realizes that the arc reactor biologically built into his chest used to power his suit is also poisoning his body. The goal is to engage students to the idea of why scientists desire to create and discover new elements. In addition, they can even see that Hollywood writers need to consult chemists in order to write scientifically valid storylines for superheroes. Instructional Prompt: Watch the video about the Science of the Avengers. Pay attention to the chemistry involved and answer the following questions. Poll the classroom for answers to the questions. Pose the following essential question, derived from the inquiry statement for the unit: How are elements synthesized both naturally and synthetically in order to understand the universe in which we live in and make technological advancements? Explore: Teacher will encourage students to work together and explain a diagram of 4 protons combining in a nuclear reaction. Ask students to predict what element would form if 4 protons to fuse together. Many students will assume that a nucleus with 4 protons would have to be the beryllium-4 nuclide, Explain: After students report out their answer, reveal the true answer that the fusion of 4 protons actually produces the helium-4 nuclide, Elaborate: Show the students the next step in the stellar nucleation, using alpha process, in which two alpha particles fuse together to form the beryllium-8 nuclide, Predicting the Products of a Nuclear Reaction Evaluate: After showing the students the first three steps of stellar nucleation, they ought to be ready to predict the rest of the cycle using the alpha process without the use of a diagram. Pay close attention to students who may confuse the superscript and subscripts. Nuclear Reactions and their Products in Stellar Nucleosynthesis Lesson: Discovering Nuclear Stability Purpose: The purpose of this lesson is to continue to give students practice with nuclear notation and counting protons and neutrons, while reviewing the limitations of the alpha process. Students will be led to discover that the alpha process is incapable of producing every element on the periodic table. This lesson will allow for the introduction of supernovae explosions and will bridge their learning from nuclear fusion to nuclear decay. Students will explore nuclear stability by graphing the number of neutrons vs. protons and calculating the slope of a group of elements using a graphing calculator. Instructional Objective: Students will be able to discuss atoms in terms of their protons and neutrons in order to graph and define the relationship between the number of neutrons and protons in the nucleus as mass number increases. Materials and Equipment: periodic tables, graphing calculators, large graph/chart paper with labeled axes (protons on x-axis, neutrons on y-axis), markers/colored pencils. Introduction: Use the following questions as a do-now activity to or kick-off into the lesson. Allow the students to share their answers with the class. Student responses will vary depending on the elements that they chose as their examples. Inform the students that they will begin to explore the relationship between the number of neutrons and protons changes as more elements are added to the periodic table. Group Activity: Divide students into groups of 3 for a total of 9 groups. Assign each group 5 elements on the periodic table. They are to plot them on a graph and calculate the slope of the line that they make. Then allow one member from each group to plot the points on a class graph. As each group adds their data points, they should see that the slope of the lines gradually increases. Procedures: List of Elements for Each Group Analysis: As students finish entering their data into a graphing software and obtain the parameters, they will answer the following analysis questions. Drawing Conclusions: The following questions are to be answered as a class after each group has appropriately labeled their points on the class graph and labeled their slopes. Lesson(s): Alpha and Beta Decay Purpose: The purpose of this lesson is to build upon the knowledge of the students’ use of nuclear notation and calculating subatomic particles by introducing alpha and beta decay. Students now know that the alpha process cannot create every element on the periodic table and they are left with a clue that some elements are created from a decay process based on the previous lesson. This lesson will look at why radioactive decay is important. The lesson on alpha and beta decay can proceed similarly to the lesson on alpha process. Below are some classic examples of beta decay. It is suggested that alpha decay and beta decay should be split into two class periods so that students can experience success in one process before moving to the next. Video and Short Discussion: This video and discussion can be used to reignite interest in radioactive decay, specifically beta decay. Post the following prompt as the kick-off activity: “Think about someone you know that has cancer, diabetes, or Alzheimer’s disease. How do you think Chemistry is related to detecting those diseases?”. Inform the students to prepare to share their answers on the condition that they feel comfortable talking about the people they know. Discussion Questions Formative Assessments Build an Atom PhET Lab: This computer simulation is now available in HTML5 meaning that a computer no longer needs to download software like Java to use it. It is a great tool for students to learn atomic structure and to visualize atomic stability before teaching the concept. The simulation also includes an option that allows for nuclear notation and the net charge of the atom for use with this unit or with future units that involve ion formation.[4] Modeling Radioactive Decay: Modeling is a great way to reinforce learning for students in the chemistry class. The Environmental Protection Agency has great resources that review the different types of radioactive decay using physical modeling.[5] Activities Noteworthy Summative Assessments Unit Tests and Quizzes: Students will be quizzed weekly, while tests are administered at the end of a unit. Both assessment methods primarily meet criterion (a), knowing and understanding, in which students must be able to explain scientific knowledge, apply scientific knowledge and understanding to solve problems set in familiar and unfamiliar situations, and analyze and evaluate information to make scientifically supported judgments. Predicting Products and Pathways in Supernovae Explosions This assessment will primarily focus on criterion B – inquiring and designing. Students will be given two scenarios. In the first scenario, students will be given a specific sequence of nuclear processes in order to determine what isotope is formed at the end of the list of processes. In the second scenario, students will be given the starting nuclide and the ending nuclide of a nuclide formed during a nuclear explosion. Students will be required to propose a mechanism. They will hypothesize the number of occurrences of each process and provide reasons why those processes should be involved in the formation of the second nuclide. They will be required to explain how to manipulate the variables and record the data. Finally, they will journal and summarize the steps involved in the figuring out the mechanism they proposed. Constructed Response Topics Criterion (d) Reflecting on the Impacts of Science: Allow students to choose an article and/or theme for a constructed response summary. Students will write a summary of the article, emphasizing the impact that the discoveries described in the chosen article has on society. Since scientific writing can drastically differ from what students learn in their English classes with the traditional five-paragraph essays, the format of the writing so be simplified to two or three paragraphs. The first paragraph should introduce the discovery or problem and its applications. The second paragraph should focus on the benefits and disadvantages or strengths and weakness of the information presented in the article. This summary can also double as a formative assessment so that students can learn how to discuss and evaluate the implications of science in regards to solving specific problems and issues. Students will be assessed in the same strands of criterion (d) in the next assessment listed. In My Element Project Purpose: The purpose of this project is assess what has already been learned in this unit while creating a bridge to the next unit on electrons. Students will take on the identity of an atom and will have to convince the class that they are the most important element in the universe. They will be required to use old and new scientific language in order to explain, discuss, and evaluate the impacts that their element has on society. Part I – Element Research Project: The element research ought to be conducted in class so that students have time to gather information and ask questions about the project as they are conducting research. Access to computers or internet technology (via library computer, school laptops, tablets, etc.) are necessary to make the most of this opportunity. Allow students 25 minutes of class time to research 3 elements that they are interested in doing. Do not allow any students to sign up to select an element until everyone has had 25 minutes of research time. This will allow students to have a backup element just in case another classmate has selected the same element prior to them. A paper sign-up sheet works best. Students should not be allowed to change elements because they should have completed enough research in 25 min to choose wisely. The format for the research project requires a physical derivative work. The suggested formats are poem, song/video, brochure/pamphlet, or a one-page graphical information sheet. Regardless of the format, there must be a written or transcribed format. For example, if you write a poem, there must be a transcription of the poem with pictures. If the format is a video, then the video either needs to be accompanied with a script or the transcription can be included in the video itself. The video option is highly encouraged for students who may be uncomfortable with public speaking or with English language learners. Since Part I of this assessment is based on primarily facts and information, it will be assessed using strands from criterion (a), knowing and understanding. Part I can also be assessed using criterion (d), reflecting on the impacts of science. Students will have to personify their element and convince classmates why they are the most important element that mankind will ever need. Students must present both the pros and the cons of their element and cite sources to support their argument. Part II – Build an Atomic Model: The second task is for students to build a 3-dimensional Bohr model of the atom. Although the Bohr model is outdated, it still reinforces the concept that protons are neutrons reside in the nucleus while the electrons are located on various energy levels. Modern atomic theory states that the electron regions have complex shapes; therefore, it is not realistic to build an atomic model established on modern atomic theory. The construction of the atomic model focuses on the organization and presentation of information in a visual aid format. Thus, this portion of the project will be assessed using strands from criterion (c), processing and evaluating. Part III – Presentations: Students will be allotted 5 minutes each to present their arguments to the class concerning the importance of their element to mankind. Classmates will evaluate their classmates on whether or not their arguments were convincing and will be required to cite evidence from the presentations to support their rating. The peer rating is necessary for managing classroom behaviors and to further enhance student learning. Essentially, the evaluation doubles as a personal set of notes with facts about each element presented. Students should not be expected to sit quietly for 25-30 presentations and become passive participants in this part of the project. This normally takes 2-3 days of classroom instruction. Preplanning Prior to the introduction of this project, students will have been introduced to the discovery of atoms and their subatomic particles. After the introduction to protons, neutrons, and electrons, students will receive the project rubric on a Monday. Students are given two days to overlook the rubric before selecting an element. On Wednesday, students will be provided with computer access to research their elements of interest for 30 minutes to determine if they can collect enough information to fulfill all the requirements of the rubric. After 30 minutes, students will be allowed to select their element on a first-come first-serve basis. Students will not be allowed to change elements unless absolutely necessary. Students are then given a week and a half to develop their project. During the week before the atomic model is due, students will engage in an in-class activity to sketch and build two-dimensional models of elements that will further enhance their understanding of the role of the subatomic particles. Thus, before the final atomic model is due, students will have been assessed on the Bohr model of the atom by quiz and a classroom activity. Addressing Diverse Learning Needs The learning needs of students are each addressed in the 3 parts of the assessment. In Part I, students must produce a writing sample in the form of a poem, song, brochure, or an information sheet. This allows for students to think creatively on how to present the information collected. Students are given the option organize their thoughts in a non-traditional, written format that can make the project more fun. Students have opportunities to personify their elements using poems and songs. In addition, students who are more comfortable with traditional presentations have the option of creating an information sheet or poster. In Part II of the assessment, visual learners can become engaged through the craftsmanship of an atomic model to demonstrate their perception of what atoms look like on the microscopic level given that no human being actually knows what an atom actually looks like. Finally, in Part III, some students are best at communicating information orally rather than through artwork or written expression. Thus, there is plenty of opportunity for students to demonstrate their understanding of atoms outside of the traditional paper and pencil test. In addition, research has shown that developing performance-based assessments with rubrics are a way of improving equity among students, specifically with English-language learners. Siegel argues that rubrics clearly inform students what is expected of them as well as they cause the teacher to focus on the equity in its design.[9] The rubric can actually serve as a feedback tool for teachers consider modifications of the rubric, which further promotes equity. The rubric also sets up the scaffold for using scientific language to support student learning. Since this assessment is heavily focused on the subatomic particles and their functional roles, this project shows all students the context of the language that is being required, regardless if the student is an ELL or a general education student. Post-Project Learning Upon successful completion of the project, students will have a gained an introduction to the periodic table of elements that will allow them to relate to the elements on a personal as well as a basic level before engaging into more complex structure/function relationships between the three types of subatomic particles. Prior to the conclusion of this unit, students will have acquired understanding of 60% of the requirements demanded in the rubric, giving the students opportunity to visually demonstrate the basics of protons, neutrons, and electrons. Their research will extend their introductory knowledge of electrons, such as the number of electrons in each shell. Thus, students’ completion of their project could propel them into higher levels of engagement and seeking understanding of the elements on the periodic table in the future. Also, since students are responsible for the presentation of their individual elements, this will promote healthy student-centered learning as the students are given the opportunity to educate peers while the teacher simply facilitates. Finally, given that this performance-based assessment marks the beginning of which students will begin to dive into the deeper aspects of chemistry, this assessment helps students to identify forthcoming vocabulary and scientific terms that need to be translated and retained throughout the remainder of the course. The project deadlines may be adjusted according to your curriculum needs. For example, the project deadline can occur at the conclusion of this unit, in the midst of the second atomic structure unit focusing on electrons, or at the end of the second unit after electrons have been thoroughly covered. Isotopes Formed in Supernovae Explosions Students will be provided with a list of isotopes formed in supernovae explosions and their decay products. Students will be required to identify the processes and the frequency of their occurrences to determine nuclear pathway by which the SNE isotope becomes a stable product. The following table includes a lists of nuclides that could be used in the assessment. This activity can be assessed using criterion (a) and criterion (b). [1] Ventuno Art. (2015). How to Fold an DIY : Origami 3D Cube. Retrieved August 18, 2016, from https://www.youtube.com/watch?v=337QxhfpY4w [2] ACS Reactions. (2015). The Science of the Avengers. Retrieved August 17, 2016, from https://www.youtube.com/watch?v=Gr3ov7R89Xo [3] NIBIB gov. (2013). How Does a PET Scan Work? Retrieved August 17, 2016, from https://www.youtube.com/watch?v=GHLBcCv4rqk [4] Build an Atom. (n.d.). Retrieved August 17, 2016, from https://phet.colorado.edu/en/simulation/build-an-atom [5] Radiation Education Activities. (n.d.). Retrieved August 17, 2016, from https://www3.epa.gov/radtown/educational-materials.html [6] Michigan State University. (2008, May 9). Designer Isotopes Push The Frontier Of Science. ScienceDaily. Retrieved August 17, 2016 from www.sciencedaily.com/releases/2008/05/080508164631.htm [7] National Superconducting Cyclotron Laboratory at Michigan State University. “Researcher Nabs Doubly Magic Tin Isotope, A North American First.” ScienceDaily. ScienceDaily, 24 December 2008. www.sciencedaily.com/releases/2008/12/081210145307.htm [8] Michigan State University. “Physicists Peer Closely At Radioactive Decay Of A Rare Isotope At The Edge Of Nuclear Existence.” ScienceDaily. ScienceDaily, 15 November 2007. www.sciencedaily.com/releases/2007/11/071108155357.htm [9] Siegel, M. (2014). Developing Preservice Teachers’ Expertise in Equitable Assessment for English Learners. Journal of Science Teacher Education, 25(3), 289-308. doi: 10.1007/s10972-013-9365-9 [10] Possibility of life origin on radioactive source of energy after our supernova explosion. (n.d.). August 17, 2016, http://dx.doi.org/10.1117/12.375080

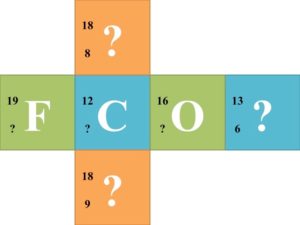
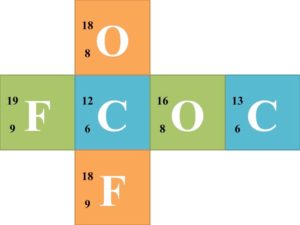
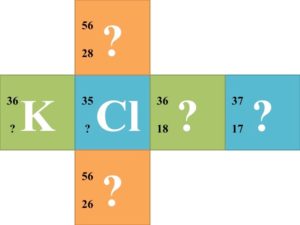
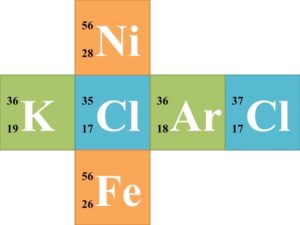
![]() Each group of 6 should try to solve for each missing component of the nuclear symbol. Once each group finishes the 6 nuclear symbols, they should trade cubes with another group. Have the groups continue trading until every group has completed the symbols of every other group.
Each group of 6 should try to solve for each missing component of the nuclear symbol. Once each group finishes the 6 nuclear symbols, they should trade cubes with another group. Have the groups continue trading until every group has completed the symbols of every other group.
![]() .
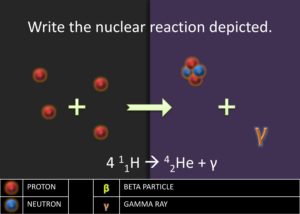
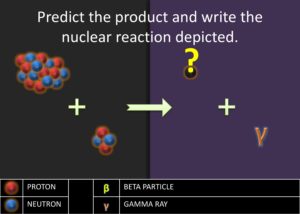
.![]() . Ask the students what could have happened to the 2 protons. Students ought to respond saying that two protons must have become neutrons. Afterward, introduce the students to gamma emission, which is the energy released as photon when a proton transforms into a neutron. Explain to students that this process is the beginning of stellar nucleation (star creation) and that the first step of stellar nucleation produces the alpha particle, which is key in the alpha process. Model for the students how to write a nuclear reaction equation using the four protons.
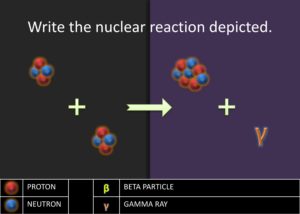
. Ask the students what could have happened to the 2 protons. Students ought to respond saying that two protons must have become neutrons. Afterward, introduce the students to gamma emission, which is the energy released as photon when a proton transforms into a neutron. Explain to students that this process is the beginning of stellar nucleation (star creation) and that the first step of stellar nucleation produces the alpha particle, which is key in the alpha process. Model for the students how to write a nuclear reaction equation using the four protons.![]() . The equation is as follows:
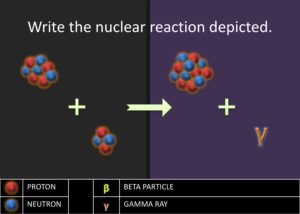
. The equation is as follows: ![]() . Then ask to students to write the nuclear chemical reaction of the nuclides involved in the diagram. Have the students report out their answer and explain why they believe the nuclides they have written are what matches in the diagrams. Challenge the students to write a rule or journal the steps involved in writing the nuclear notation for nuclides produced in the alpha process. Students should have a procedure similar to the following steps:
. Then ask to students to write the nuclear chemical reaction of the nuclides involved in the diagram. Have the students report out their answer and explain why they believe the nuclides they have written are what matches in the diagrams. Challenge the students to write a rule or journal the steps involved in writing the nuclear notation for nuclides produced in the alpha process. Students should have a procedure similar to the following steps:
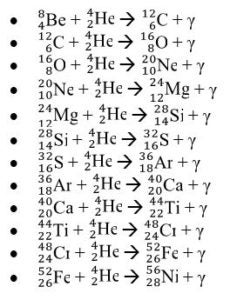

List of Isotopes and their Products from Supernovae Explosions[10]
SNE Isotope
Product Isotope
Decay Particles
41Ca
41K
Electron capture
60Fe
60Ni
2 beta emissions
26Al
26Mg
1 positron emission
237Np
209Bi
7 alpha emissions, 4 beta emissions
53Mn
53Cr
Electron capture
129I
129Xe
1 beta emission
236U
232Th
1 alpha emission
235U
207Pb
7 alpha emissions, 4 beta emissions
238U
206Pb
8 alpha emissions, 6 beta emissions
Teacher Resources Student Resources
Appendix – Key Vocabulary and Terms Appendix – Isotope Puzzle Cube Samples Appendix – Sample Slides for Modeling Alpha Process Appendix – Discovering Nuclear Stability Student Handout Template Appendix – Discovering Nuclear Stability Student Handout Template, p2 Appendix – Example Assessment Rubric Criterion A: Knowing and understanding For use with In My Element Project Maximum: 8 points i. state scientific knowledge. i. outline scientific knowledge. · # of subatomic particles · Group, Period, Block Location, and Group Name i. describe scientific knowledge. · Valence electrons · Neutron/proton ratio · Pictures or drawing of element · Describing uses of element i. explain scientific knowledge. · Relative particle sizes · Explaining uses of element (Why is element important?) Appendix – Example Assessment Rubric Criterion C: Processing and evaluating For use with In My Element Project Maximum: 8 points i. collect and present data in numerical and/or visual forms. · Poor Craftsmanship i. correctly collect and present data in numerical and/or visual forms. · Good craftsmanship i. correctly collect, organize, and present data in numerical and/or visual forms. · Solid craftsmanship i. correctly collect, organize, transform, and present data in numerical and/or visual forms. · Relative particle sizes · Superior craftsmanship Appendix – Example Assessment Rubric Criterion D: Reflecting on the impacts of science For use with In My Element Project Maximum: 8 points i. outline the ways in which your element is applied and used to address a specific problem or issue ii. outline the implications of using your element and its application to solve a solve a specific problem or issue, interacting with a factor iii. apply scientific language to communicate understanding but does so with limited success iv. document sources, with limited success. i. summarize the ways in which your element is applied and used to address a specific problem or issue ii. describe the implications of using your element and its application to solve a solve a specific problem or issue, interacting with a factor iii. sometimes apply scientific language to communicate understanding iv. sometimes document sources. i. describe the ways in which your element is applied and used to address a specific problem or issue ii. discuss the implications of using your element and its application to solve a solve a specific problem or issue, interacting with a factor iii. usually apply scientific language to communicate understanding clearly and precisely iv. usually document sources. i. explain the ways in which your element is applied and used to address a specific problem or issue ii. discuss and evaluate the implications of using your element and its application to solve a solve a specific problem or issue, interacting with a factor iii. consistently apply scientific language to communicate understanding clearly and precisely iv. document sources completely. Appendix – MYP Command Terms

Student Copy
Teacher Copy



Achievement level
Level descriptor
Project requirements
0
The student does not reach a standard identified by any of the descriptors below.
1–2
The student is able to:
· Element Name, Atomic Number, Atomic Symbol, Atomic Mass
3–4
The student is able to:
· Atomic Mass with units
5–6
The student is able to:
· 4 physical or chemical properties
7–8
The student is able to:
· Correct neutron/proton ratio with units.
Achievement level
Level descriptor
Project requirements
0
The student does not reach a standard identified by any of the descriptors below.
1–2
The student is able to:
· incorrect # of subatomic particles
3–4
The student is able to:
· Correct # of subatomic particles
5–6
The student is able to:
· Correct placement of subatomic particles
7–8
The student is able to:
· Correct neutron/proton ratio with units.
Achievement level
Level descriptor
0
The student does not reach a standard identified by any of the descriptors below.
1–2
The student is able to:
3–4
The student is able to:
5–6
The student is able to:
7–8
The student is able to:
Command term
Definition
Analyze
The student does not reach a standard identified by any of the descriptors below.
Apply
Use knowledge and understanding in response to a given situation or real circumstances. Use an idea, equation, principle, theory, or law in relation to a given problem or issue.
Describe
Give a detailed account or picture of a situation, event, pattern, or process.
Discuss
Offer a considered and balanced review that includes a range of arguments, factors, or hypotheses. Opinions or conclusions should be presented clearly and supported by appropriate evidence.
Document
Credit sources of information used by referencing (or citing), following one recognized referencing system. References should be included in the text and also at the end of the piece of work in a reference list or bibliography.
Evaluate
Make an appraisal by weighing up the strengths and limitations.
Explain
Give a detailed account including reasons and causes.
Organize
Put ideas and information into a proper or systematic order
Outline
Give a brief account or summary.
Present
Offer for display, observation, examination, or consideration.
State
Give a specific name, value, or other brief answer without explanation or calculation.
Summarize
Abstract a general theme or major point(s).
Transform
Involves processing raw data into a form suitable for visual representation. This process may involve, for example, combining and manipulating raw data (by adding, subtracting, squaring or dividing) to determine the value of a physical quantity and also taking the average of several measurements. It might be that the data collected are already in a form suitable for visual representation–in the case of the distance travelled by a woodlouse, for example. If the raw data are represented in this way and a best-fit line graph is drawn the raw data have been processed.
Pennsylvania Core Academic Standards Nuclear Chemistry · Describe the process of radioactive decay by using nuclear equations and explain the concept of half-life for an isotope. · Compare and contrast nuclear fission and nuclear fusion. Atomic Theory · Describe Rutherford’s “gold foil” experiment that led to the discovery of the nuclear atom. Identify the major components (protons, neutrons, and electrons) of the nuclear atom and explain how they interact. Scientific Investigations · Know that both direct and indirect observations are used by scientists to study the natural world and universe. · Identify questions and concepts that guide scientific investigations. · Formulate and revise explanations and models using logic and evidence. · Recognize and analyze alternative explanations and models. · Examine the status of existing theories. · Evaluate experimental information for relevance and adherence to science processes. · Judge that conclusions are consistent and logical with experimental conditions. · Interpret results of experimental research to predict new information, propose additional investigable questions, or advance a solution. · Communicate and defend a scientific argument. District of Philadelphia Standards 3.1.10.B.2: Examine the advantages of using models to demonstrate processes and outcomes (e.g., blue print analysis, structural stability). 3.1.10.B.3: Apply mathematical models to science and technology. 3.4.10.A.7: Describe various types of chemical reactions by applying the laws of conservation of mass and energy. 3.4.12.A.3: Explain how radioactive isotopes that are subject to decay can be used to estimate the age of materials. 3.4.12.A.6: Apply the conservation of energy concept to fields as diverse as mechanics, nuclear particles and studies of the origin of the universe. 3.4.12.A.8: Quantify the properties of matter (e.g., density, solubility coefficients) by applying mathematical formulas. 3.8.12.C.3: Analyze and communicate the positive or negative impacts that a recent technological invention had on society. 3.8.12.C.4: Evaluate and describe potential impacts from emerging technologies and the consequences of not keeping abreast of technological advancements (e.g., assessment alternatives, risks, benefits, costs, economic impacts, constraints). Next Generation Science Standards HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials. Common Core Standards HSN-Q.A.1 Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays. (HS-PS1-3),(HS-PS1-8),(HS-PS2-6) HSN-Q.A.2 Define appropriate quantities for the purpose of descriptive modeling. (HS-PS1-8),(HS-PS2-6)
Anchor Descriptor
Core Standard
3.2.C.A3
· Identify the three main types of radioactive decay and compare their properties.
3.2.C.A5
· Recognize discoveries from Dalton (atomic theory), Thomson (the electron), Rutherford (the nucleus), and Bohr (planetary model of atom), and understand how each discovery leads to modern theory.
3.2.C.A6
· Compare and contrast scientific theories.
Anchor Descriptor
Core Standard
3.1.10.B: Describe concepts of models as a way to predict and understand science and technology.
3.1.10.B.1: Distinguish between different types of models and modeling techniques and apply their appropriate use in specific applications (e.g., kinetic gas theory, DNA).
3.1.10.C: Apply patterns as repeated processes or recurring elements in science and technology. concepts of models as a way to predict and understand science and technology.
3.1.10.C.1: Examine and describe recurring patterns that form the basis of biological classification, chemical periodicity, geological order and astronomical order.
3.4.10.A: Explain concepts about the structure and properties of matter
3.4.10.A.1: Know that atoms are composed of even smaller sub-atomic structures whose properties are measurable.
3.4.10.D: Explain essential ideas about the composition and structure of the universe.
3.4.10.D.3: Describe the nuclear processes involved in energy production in a star.
3.4.12.A: Apply concepts about the structure and properties of matter.
3.4.12.A.2: Classify and describe, in equation form, types of chemical and nuclear reactions.
3.4.12.D: Analyze the essential ideas about the composition and structure of the universe.
3.4.12.D.1: Analyze the Big Bang Theory?s use of gravitation and nuclear reaction to explain a possible origin of the universe.
3.6.10.A: Apply biotechnologies that relate to propagating, growing, maintaining, adapting, treating and converting.
3.6.10.A.2: Apply knowledge of biomedical technology applications in designing a solution to a simple medical problem (e.g., wheel chair design, artificial arteries).
3.8.12.A: Evaluate the consequences and impacts of scientific and technological solutions.
3.8.12.C.2: Analyze scientific and technological solutions through the use of risk/benefit analysis.
Anchor Descriptor
Core Standard
HS. Structure and Properties of Matter
HS-PS1-8. Develop models to illustrate the changes in the composition of the nucleus of the atom and the energy released during the processes of fission, fusion, and radioactive decay.
HS. Waves and Electromagnetic Radiation
HS-PS4-5. Communicate technical information about how some technological devices use the principles of wave behavior and wave interactions with matter to transmit and capture information and energy.
Anchor Descriptor
Core Standard
ELA/Literacy
RST.9-10.7 – Translate qualitative or technical information expressed in words in a text into a visual form and translate information expressed visually or mathematically into words.
Mathematics
MP.2 – Reason abstractly and quantitatively. (HS-PS4-1), (HS-PS4-3)