The plasma membrane is a thin barrier that separates the inside of the cell from its external environment. Through repeated experiments and advances in technology, scientists have developed the “Fluid Mosaic Model” to describe the structure of this dynamic membrane. Because of its unique structure, the plasma membrane can effectively regulate what enters and exits the cell. Students need to understand how the plasma membrane works in order to fully grasp the concepts the come later on.
This unit is designed to teach 9th and 10th grade biology students about the content using general principles of scientific processes learned in the beginning of the year. Students will explore the plasma membrane and cellular transport through the eyes of a scientist learning about this topic for the first time. Students will plan and carry out experiments, analyze and interpret data, and evaluate and communicate the information learned.
The fluid mosaic model of the plasma membrane developed as a result of evidence gathered from conceptual and technological advances. In the late 1800s, scientists initially hypothesized that membranes must be made of lipids, after observing that substances dissolved in lipids entered cells more quickly than those insoluble in lipids (Campbell 138). After experiments with red blood cells, two Dutch scientists, E. Gorter and F. Grendel, proposed that membranes must actually be a phospholipid bilayer, two molecules thick. This bilayer would provide a stable boundary between the aqueous environments inside and outside the cell.
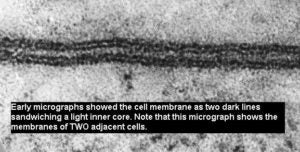
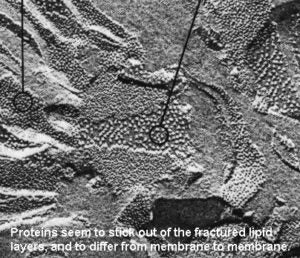
With the development of the electron microscope, freeze-fracture methods and additional studies with capillaries, scientist were able to further conclude that proteins were found embedded within the plasma membrane (integral proteins) or along the sides (peripheral protein).
Continued research eventually led to the most current model of the plasma membrane, one that is composed of a phospholipid bilayer embedded with cholesterol molecules, cytoskeleton, and long carbohydrate chains, in addition to protein molecules. The phospholipid bilayer contains both hydrophobic and hydrophilic regions, as do many of the integral proteins themselves.
The plasma membrane plays a role in structural support, cell to cell communication and cell identification. However, its’ main function is to controls what enters and exits the cell. Because of its unique structure, only certain substances are allowed to freely enter and exit the cell. For this reason, the plasma membrane is “semi-permeable”.
Passive cellular transport is diffusion, the tendency for molecules to spread out into the available space, across a membrane (Campbell 145). Molecules will move down its concentration gradient, to areas of lower concentration, in order to reach equilibrium. Some small, non-polar molecules (such as carbon dioxide and oxygen gas) can simply pass through the plasma membrane. Other small, polar substances (such as sodium and potassium ions) must pass through a protein channel in order to enter or exit the cell (Holt 80).
The passive transport of water across the plasma membrane is called osmosis. In osmosis, water moves towards areas of higher solute concentration because the solute particles are too large in size to move across the membrane. Cells can be found in one of three solutions in varying concentrations. Hypertonic solutions contain more solute than the cell itself. If a red blood cell is placed in a hypertonic solution, for example, water will move out of the cell and into the solution, causing the cell to decrease in size. Hypotonic solutions contain a lower solute concentration than the cell. Isotonic solutions are those equal solute concentration as the cell (Campbell 146).
Students often have difficulty understanding this portion of the biology curriculum because the vocabulary is new, the content is dense and they often struggle to relate the information to their own background knowledge. Explicit teaching of the plasma membrane and cellular transport leads to rote memorization of facts, which tend to be quickly forgotten moments after a unit assessment. Student learning must be an active process that connects to problems relevant to students’ lives (Chaplin and Manske, 2005). By challenging students to make connections beyond just words on a page, they will have better memory recall of how the plasma membrane works, which will then help them understand the concepts that come later (i.e. how glucose enters the cell in cellular respiration, how viruses are able to recognize and attach to particular cells, how eggs and sperm fuse during fertilization).
The objectives of the unit will include the following:
This unit will include a variety of lessons that will cater to different learning modalities. The unit will start with inquiry based lessons and activities that will guide students to draw their own conclusions about plasma membrane structure and function. Instead of a teacher-centered lecture about the plasma membrane composition, students will be given data from early experiments and asked to think about what this information tells us about the membrane. Based on the information learned, students will build a 3D model of the plasma membrane in structured groups of 3-4. After students understand the membrane structure and composition, they will conduct a series of experiments exploring osmosis and cellular transport. Through a series of guided questions and observations, students will discover on their own how substances are moved across the membrane. Teachers reinforce the major points of cellular transport after the experiments are complete. Finally, students will apply their knowledge by working in small groups to complete a case study on osmosis.
The proposed unit is for a ninth grade biology course. The class meets every day for 51 minutes. At Horace Furness High School, science classes have access to a lab room and full-time lab assistant one day a week. Students should have sufficient background knowledge on water properties (including polarity, hydrophobic/hydrophillic terminology) and macromolecules (especially phospholipids and proteins) going into this unit. Lesson One – Objective: Correctly state the components of the plasma membrane and describe/show how they are organized in the plasma membrane – Activities: Lesson Two – Objective: Construct the most current fluid mosaic model of the plasma membrane using a variety of supplies. – Activities: Lesson Three: Objective: (1) Analyze the relationship between structure and function of the plasma membrane (2) Determine what factors will impact whether a substance will pass through a membrane Activities: Lesson Four: Objective: Determine what factors will impact whether a substance will pass through a membrane Activities: Lesson Five: Objective: Explain mechanisms organisms use to maintain homeostasis Activities: Lesson Six: Objective: Write informative/explanatory texts to examine and convey complex ideas, concepts, and information clearly and accurately Activities: Lesson Seven: Objective: Explain mechanisms organisms use to maintain homeostasis Activities: The unit will culminate in a written exam in which students demonstrate their knowledge by answering multiple choice and free response questions. Many of these questions will be pulled directly from a sample high-stakes exam (i.e. Biology Keystone, Biology SAT, ACT Science and/or Science PSSA).
Student Resources Doherty, Jennifer, and Ingrid Waldron. “Diffusion Across a Selectively Permeable Membrane.” Hands-on Activities for Teaching Biology to High School or Middle School Students. N.p., n.d. Web. 14 June 2013. [This is a very clear and straightforward passive transport lab handout for students.] Johnson, George B. Holt Biology. Orlando, Fl.: Holt, Rinehart and Winston, 2004. Print. [This biology textbook provides a good reference and pictures for students.] “Lesson B: Cell Membranes.” Nuffield Foundation Teaching About Science. N.p., n.d. Web. 05 Mar. 2013. [This website provides experimental data on the discovery of the Fluid Mosiac Model of the plasma membrane.] Nash, Troy. “Osmosis Is Serious Business.” National Center for Case Study Teaching in Science (NCCSTS). N.p., n.d. Web. <http://sciencecases.lib.buffalo.edu/cs/files/osmosis.pdf>. [An interesting case study relating osmosis to plant and human health.] Teacher Resources Campbell, Neil A., and Jane B. Reece. Biology. San Francisco: Benjamin Cummings, [This college biology textbook is a great resource on all science topics.] Chaplin, Susan B. “A Theme-Based Approach to Teaching Non-Majors Biology.” NSTA.org. N.p., Sept. 2005. Web. [This journal article describes another approach to making science learning more relevant to students.] Models of the Plasma Membrane. N.p., n.d. Web. <http://www.hsc.on.ca/moffatt/bio3a/cellbio/phase1.htm>. [This website outlines how the models of the plasma membrane have changed over time.] Perlstein, Ethan. “Insights into Cell Membranes via Dish Detergent.” TEDEducation, n.d. Web. 30 Mar. 2013. [This video is a great introduction to the composition of cell membranes.] Pike, Angela. “Build A Model of the Cell Membrane.” Education.com. N.p., n.d. Web. <http://www.education.com/science-fair/article/build-cell-membrane-model/>. [This building activity is a great way for students to visualize the cell membrane.] Classroom Materials Additional materials required for this unit include: – Cell membrane model supplies per group of 3-4 students: 50 cotton swabs, rubber band (to hold the cotton swabs together), pipe cleaners, straws cut in half, tiny twizzler candy pieces, one marble and one grain of rice – A list of supplies needed for “Diffusion Across A Selectively Permeable Membrane” can be found at: http://serendip.brynmawr.edu/sci_edu/waldron/pdf/MembraneTeachPrep.pdf – Egg Cell Lab supplies per group of 3-4 students: 2 eggs, 2 plastic cups, 1 toothpick, masking tape (to label the plastic cups), vinegar and corn syrup.
Handout One: “Lipid Layer Evidence” Front page: Background: Data from the experiment which laid the foundations for a model of membrane structure is summarized in the table below. Gorter and Grendel obtained the membranes of red blood cells. They calculated the area of the red blood cell membrane and then extracted the lipids that were present. These lipids were dissolved in petroleum ether and allowed to spread into a layer one molecule thick on a surface of water and the area was measured. **Remember that “surface area” is the total area of a 3D object. Directions: Divide ‘B’ by ‘A’ to determine the ratio of total surface area to lipid surface area. Then, answer the analysis questions that follow. B/A Analysis Questions: [ 1 2 3 ] molecules thick. Back page: Handout Two: Fluid Mosaic 3D Model Follow the following procedure to make a model of the plasma membrane. When you come across a question, answer it in complete sentences. Handout Three: “Modeling Passive Transport with an Egg Cell Lab Guidelines” Introduction: The purpose of this lab is to soak a shell-less egg in various liquids overnight and observe how the size of the egg changes. The shell-less egg will represent a cell and its selectively permeable membrane. Eggshells are made up of the mineral calcium carbonate, which dissolves in acids such as vinegar. Your eggs have been soaked in vinegar overnight. After the shell has been dissolved, only the membrane will remain around the egg. Pre-Lab Questions: Table 1: Analysis Questions:
Animal
Total surface are of the red blood cell (A)
Surface area occupied by the lipids extracted (B)
Ratio
Dog
31.3
62
6.2
12.2
Sheep
2.95
6.2
2.65
5.8
Rabbit
5.46
9.9
5.46
8.8
Guinea-pig
0.52
1.02
0.52
0.97
Goat
0.33
0.69
0.33
0.63
Man
0.47
0.92
0.47
0.989
Liquid
Beginning mass (g)
Mass after soaking (g)
Observations (written and pictorial)
Percent Mass Change
Corn syrup
Vinegar (5% acetic acid, 95% distilled water)
This unit aligns to the Pennsylvania Academic Standards for Science and Technology and Engineering Education. 3.1.A