Author: Joseph L. Hill, Jr.
School/Organization:
High School of the Future
Year: 2015
Seminar: Penn Laboratory on Energy, Sustainability and Environment
Grade Level: 11
Keywords: batteries, battery, Danielson, Electrochemistry, lithium ion, oxidation, reduction, voltaic cell
School Subject(s): Chemistry
This past March 2015, the School District of Philadelphia released the blueprint for its future success titled Action Plan 3.0.[1] It is a description of the District’s current and planned work priorities. According to the foreword called “Superintendent’s Message”, we are employed in a time of diminishing resources which require a greater commitment to equity. The commitment to equity which the District has committed itself to in regard to the evaluation of Educators is the Danielson Framework for Teaching.[2] Danielson’s Framework is not discipline specific so it applies to all subjects. It is propositioned upon what Danielson calls The Four Domains Of Teaching Responsibility. These domains are:
| Domain 1 – Planning and Preparation | Domain 2 – The Classroom Environment |
| Domain 3 – Instruction | Domain 4 – Professional Responsibilities |
Too often, Educators labor over lesson plans and curriculum units which recite at length, all the wonderful learning task our learners will experience once a curriculum unit is complete and we forget that the School District of Philadelphia is not evaluating us on the platform of learner achievement via an individual curriculum unit.
The purpose of this curriculum unit is to make you; the School District of Philadelphia Educator meet the third of four action goals specified in Action Plan 3.0 – and that is 100% of schools will have great principals and teachers. To be considered a great Educator, you will have to be highly rated on a scale of 1 – 4 in the four domains described by Danielson. This unit will focus on Domain 2 (The Classroom Environment) in addition to specific learner outcomes. Domain 2 is primarily concerned with observable evidence that learners are able to work independently of educators as evidenced by educator interactions with learners and learners’ interactions with other learners. The tactics we will use to achieve our goals will be lessons based on oxidation, reduction and electrochemistry. It is my intention to captivate the learners by making connections between the lessons on redox reactions and the chemistry of their cell phone batteries.
The overriding idea is that if you implement this curriculum unit, you will demonstrate to an observing ratings officer that you have carefully planned and prepared a unit geared towards the success of your learners and that the instructional climate in which you deliver the Instruction (Domain 2) is exemplary! Throughout the writings of this curriculum unit, I will emphasize tactics and techniques geared towards satisfying the requirements of Danielson’s Framework For Teaching and, ultimately, securing you a higher score on your annual teacher evaluation which is based upon Danielson.
[1] http://webgui.phila.k12.pa.us/uploads/bi/6T/bi6T3DCFvqvaCSoHeGZoZg/Action-Plan-3.0-FINAL-3-4-15.pdf
[2] Danielson, Charlotte. Enhancing Professional Practice: A Framework for Teaching. 2nd ed. Alexandria, VA: Association for Supervision and Curriculum Development, 1996. Print.
Download Unit: Hill-Joseph-unit.pdf
Did you try this unit in your classroom? Give us your feedback here.
Educators have been taught that learners would be more inclined to take an interest in learning the subject matter at hand if one can make real-world connections.[1] It seems that the only time learners are not captivated by their electronic devices is when the power has run out. In order to maintain our constant connectivity with the world around us, cellphones have to have a reliable, mobile energy storage device. Consumer demands require that it be lightweight, long-lasting, safe and rechargeable. Regardless of the mobile carrier, phone name or screen size, underneath it all is chemistry! All of the energy supplied to the modern cell phone is generated using electrochemical cells. Through the processes of oxidation and reduction in the cells, an electric current is generated. Electrochemical cells used in this way are better known to most people as batteries. Learners will be introduced to the processes of oxidation and reduction (Redox reactions). How to recognize redox reactions and to balance them using half-reactions. They will learn the basic components of electrochemical cells and how to calculate the voltage of a cell. The four main types of redox reactions are synthesis (combination), decomposition, displacement (single or double), and combustion. The School District of Philadelphia Unit 6 Chemical Reactions focuses on using chemical equations to describe the many types of chemical reactions that can occur. Chemical equations provide information about chemical reactants and the products they form. The overriding goal of this prescribed SDP unit is that learners will collaborate with a team as they learn to identify and name different types of chemical reactions. They will learn to represent reactions in balanced chemical equations. Assessed prior knowledge should confirm that learners are familiar with the following topics: Learners most appropriate for this unit are in eleventh grade Honors chemistry. This unit will satisfy many of the School District of Philadelphia teaching goals, listed above, by focusing on Lithium-ion batteries. We will explore the history its development, explain energy density and why lithium-ion batteries dominate modern cellphones. The unit culminates with a study of the true cost of modern cellphones with learners proposing plans to mitigate damage to the ecosystem through the responsible disposition of used cellphones. [1] https://www.commonsensemedia.org/educators/blog/for-stem-learning-real-world-application-matters
This unit will introduce learners to the importance of a class of chemical reactions called oxidation-reduction or REDOX for short. This is followed by unit activities designed to give learners a deeper, more connected understanding of batteries and electrochemistry. The final study is of electrochemistry’s connection to our everyday lives through the examination of Lithium-ion batteries and its ubiquitous use in cellphones. Pennsylvania standard CHEM.B.2.1[1] describes successful learners as being able to predict what happens during a chemical reaction. To accomplish this, they will learn about the processes of oxidation and reduction, how to assign oxidation numbers to atoms, and recognize redox reactions. They will then learn the basic components of the electrochemical cell. Learners will meet Pennsylvania standard CC.3.5.11-12.C[2] by conducting a two-part laboratory investigation. First, a simple wet-cell battery using fruit will be examined. Secondly, a voltaic pile will be created to explore how stacking smaller cells can produce appreciable voltage and power. Several concepts will be investigated, including, but not limited to batteries, oxidation-reduction, and electrochemical cells (voltaic cells, galvanic cells). To ensure learners have satisfied the Next Generation Science Standard HS-PS1-2[3] and made the connection between our formal study of electrochemistry and the Lithium-ion cell battery which powers their cellphone, learners will be tasked with explaining why the Lithium ion is the preferred element for cellphone battery use as opposed to another group 1 Alkali metal. Finally, to meet the Pennsylvania Core Standard CC.3.5 learners will author an informational/explanatory essay which considers the real-world implications of improper disposal of unwanted Lithium-ion batteries and propose actionable behaviors to minimize harming our ecosystem. [1] http://www.pdesas.org/standard/StandardsBrowser/76626 [2] http://www.pdesas.org/standard/StandardsBrowser/162528 [3] http://www.nextgenscience.org/hsps1-matter-interactions
Assessing Prior Knowledge: Learners should be familiar with the following topics: electronegativity, the mole, balancing equations, the activity series, the nature of solutions and ionization. Misconception alerts include making sure learners do not define oxidation as a reaction in which a substance gains oxygen or loses hydrogen and defining reduction as a reaction in which a substance loses oxygen or gains hydrogen. Instead, the more accurate and generally accepted definitions describe the gain or loss of electrons. A mnemonic will be taught that learners will find helpful in remembering the more accurate definition of oxidation and reduction. Danielson domain 2 is concerned with learners being able to ask and answer questions independently of the educator. According to this model, superior classrooms will show evidence of learners posing questions to each other. To assist in the development of this environment, create a parking lot. A parking lot is a space where learners and educators can post questions that occur during lessons which may eventually be answered by people in the learning group. Educators should model the posting of questions to the parking lot and continuously encourage learners to do the same.
Lesson One: Introduce oxidation and reduction, how to assign oxidation numbers to atoms, how to identify redox reactions, and how to balance them using the half-reaction method Prior to engaging learners, have them write a short list of all the things they already know or think they know about electrochemistry. Then have them list the things they might want to know about electrochemistry. Questions such as, what are the components of various types of batteries, can be used to instigate the conversations. Group Activities will be incorporated to allow learners to investigate the real-world connection of oxidation-reduction chemistry to their daily lives. Oxidation-reduction reactions may initially seem uncommon to learners, yet they occur all around them. Learners will work in small groups to research four naturally occurring redox reactions or redox reactions that are useful for humans. They will write the equations for these reactions on poster boards and use half-reaction methods to balance them. Additionally, learners can illustrate their reactions with photographs and drawings – the posters can be displayed around the room. Demonstrations such as making a fruit battery and other lab activities which generate electrical current will also be incorporated. Lesson Two: What does electrochemistry have to do with my cell phone? The average learner is familiar with the fact that metal wires, particularly copper, are used to conduct electricity. Our homes, schools and businesses are wired with metal cables that conduct electricity which is used for power and communication needs. Pose the following question to your learners on the parking lot and encourage them to develop questions of their own for display there as well. Can pure water or aqueous solutions of salt or sugar conduct an electric current? A simple demonstration to answer this question can be conducted as follows: Pour 100 ml of distilled water into each of three 250 ml beakers. Label the first beaker A, the second B, and the third C. Place a 5 grams of salt into beaker B, and 5 grams of sugar into beaker C. A simple test for conductivity can be made by placing copper wire electrodes, batteries, and a flashlight bulb or light-emitting diode in series[1] as shown below. Repeat this setup for beaker C. You will immediately observe that the lamp will not light up in either the distilled water (beaker A) or sugar solution (beaker C), but it will light up in the saline solution (Figure B). It should be clear from this activity that the salt water conducts electricity. With respect to Danielson’s domain 2, educators should stop at this point and require learners to post any question which comes to their minds to the parking lot. Remember, one of our goals is to develop a classroom where learners being asking and answering questions amongst themselves. After giving your learners ample time to develop questions and post them, go over to the parking lot and read the posted questions. Hopefully someone has asked the following question. How is current conducted without metal wires? If no one has asked this question, try to twist the nearest question into a statement similar to the one I suggested. This will encourage learners to continue their independent thinking which is observable by administrators monitoring your learning environment. Tell the learners that by conducting a series of electrochemical investigations, they will find for themselves, the answers to this and many more questions. Electrochemistry is the study of the interchange of chemical and electric energy. There are primarily two processes in electrochemical, both involving oxidation-reduction (redox) reactions. One process is the generation of an electric current from a chemical reaction, and the other is the use of an electric current to produce a chemical reaction. A good example of the first process is the production of a current from a battery to light a lamp. An example of the second process is the use of an electric current to separate water into hydrogen and oxygen gas in a process known generically as electrolysis.[3] Typically, a redox reaction occurs when an oxidizing agent contacts a reducing agent. For example, when placing a strip of zinc metal into a solution of copper sulfate, zinc atoms lose electrons and enter the solution while copper ions gain electrons and are deposited as metallic copper onto the zinc strip. In this redox reaction there is a direct transfer of electrons from one substance to another. Here is where it gets interesting, if the oxidizing agent (copper in our example) is physically separated from the reducing agent (zinc in our example) the transfer of electrons can be forced to take place through an external conducting medium such as a copper wire, and the resulting electric current can be used to perform useful work. Work (W) is defined as the product of the force (F) required to move an object and the distance (d) the object is moved (W =Fd). Work is required when you carry your heavy lesson plans from the first floor to the second floor of your school since the heavy lesson plans on the second floor is at a higher energy level (possess more gravitational potential energy[4]). The difference in potential energy between the lesson plan on the second floor and the lesson plan on the first floor can be referred to as the “potential difference.” Similarly, work is needed to move electrons in a wire or to move ions through a solution to an electrode. An electric charge moves from a point of higher electrical potential (higher ‘electric pressure’) to a point of lower electric potential (lower ‘electric pressure’) just as the heavy lesson plan can fall off the second floor (possess more gravitational potential energy) onto the head of an administrator on the first floor (lower potential energy) thus doing work (no injuries occur in this scenario). “Electric potential difference determines the movement of charge just as air pressure determines the movement of weather fronts and water pressure determines the movement of water in the pipes in your home or city. Electric potential difference is the difference in electrical potential between two points, and is measured in volts. To determine the electrical work expended in moving a charge through a conductor, you must multiply the charge by potential difference: Electrical work (joules) = charge (coulombs) x potential difference (volts) A joule (J) is amount of energy required to move a coulomb © of electricity through a potential difference of one volt (V): J = CV.”[5] Day One – Electrode Potentials Investigative Concepts: Electrode potentials, oxidation-reduction, electrochemical cells (voltaic cells, galvanic cells), wet cells, batteries, electrolytes, electrodes, cathodes, anodes, reduction potential, voltage. Materials: Lemon (or other citrus fruit), tomato (or apple, potato or pineapple), strips of magnesium, aluminum, zinc, iron, nickel, tin and copper (see appendix A for inexpensive sources of various substances used in this activity), multimeter (an inexpensive analog multimeter will do but a digital multimeter is preferred because it consumes less current) Safety: Do not eat food used in this or any other experiment. Discard the fruits after the activity is completed. Principles and Procedures: In 1780, Italian anatomy professor Luigi Galvani was probing dissected frog legs when one of them spontaneously twitched. Needless to say, Galvani was quite surprised to see the leg of a frog move hours after it had died. Galvani was fascinated by this occurrence and tried to recreate the conditions under which it had occurred. After careful observation and study, he noted that the leg would contract whenever it simultaneously touched two different metals (such as and iron probe and a copper pan) that were themselves in contact with each other. In his study of anatomy, Galvani had inadvertently discovered the basics of an electrochemical (galvanic) cell, varieties of which we now use to provide electricity to start our cars, light our flashlights, run our watches, and powers numerous portable electronic devices such as cell phones! Oxidation and Reduction Occur Together In oxidation-reduction reactions electrons are transferred from reducing agents as the reactants collide. The energy of electrons exchanged in an oxidation-reduction can be tapped to perform work if the oxidizing and reduction agents are physically separated, yet connected by a conducting material. Electrons released from the reducing agent travel through the conductor to reduce the oxidizing agent. As these electrons move through the conductor, their energy may be transformed into light in a flashlight filament, heat in an electric hand warmer, sound in a buzzer of a car alarm or motion in a frog’s leg. Oxidation occurs at one end of the conductor called the anode, while reduction occurs at the other, known as the cathode. Since the reactions that take place at the electrodes are physically separated but interdependent, we can refer to them as half-reactions and the locations at which they occur as half-cells.[6] Activity 1 (45 minutes) FOCUS ACTIVITY 5 minutes Have learners list the types of reactions they have learned from previous lessons on the whiteboard. Tell them that they are going to be learning about a specific type of reaction called an oxidation-reduction reaction which includes all the other types previously learned. In short, this activity has students make a list of the different types of reactions they have learned. MOTIVATE 10 minutes In an effort to get learners interested in the coming discussions, show learners a browned wedge of apple, rusted piece of iron, and a burning candle and ask them: ANSWERS TO MOTIVATE Explain that these reactions are oxidation reactions, but not all oxidation reactions involve oxygen. TEACH 30 minutes Tell learners that oxidation involves a loss of electrons. Use the formation of sodium chloride as an example. It is formed by the transfer of electrons from sodium atoms to chlorine atoms and can be described by this reaction: 2Na(s) + Cl2(g) à 2NaCl(s) Tell the learners that in making sodium chloride, sodium is oxidized from Na to Na +. Key Terms: Oxidation – a reaction that removes one or more electrons from a substance such that the substance’s valence or oxidation state increases. Reduction Involves a Gain of Electrons Tell the learners that in the making of sodium chloride, the electrons lost by the sodium atoms do not just go away. They are gained by the chlorine atoms. The gain of electrons is described as reduction. The chlorine atoms are reduced as they change from Cl2 to2Cl–. Key Terms: Reduction – a chemical change in which electrons are gained, either by the removal of oxygen, the addition of hydrogen, or the addition of electrons Oxidation and Reduction Occur Together Remind learners that whenever oxidation occurs, reduction must also occur. Although these two reactions can take place at different places, most often they happen in a single place. A single reaction in which an oxidation and a reduction happen is called an oxidation-reduction reaction or redox reaction. On paper, learners can determine whether or not an atom is oxidized or reduced by keeping track of oxidation numbers. Key Terms: Oxidation-reduction reaction – any chemical change in which one agent is oxidized (loses electrons) and another agent is reduced (gains electrons); also called redox reaction Oxidation numbers – the number of electrons that must be added to or removed from an atom in a combined state to convert the atom into the elemental form. If the oxidation number of an atom increases during a reaction, the atom is oxidized. Likewise, if the oxidation number of the atom decreases during a reaction, it is reduced. In order for learners to determine whether or not an atom is oxidized or reduced, Educators must teach their learners to first assign oxidation numbers and then to determine oxidation numbers, here’s how: SKILLS BOX Educators can use the following example to assist learners in determining oxidation numbers as well as applying the rules for assigning oxidation numbers. Task: Assign oxidation numbers to the sulfur and oxygen atoms in the pyrosulfate ion, S2O72- Apply rule #1 Identify the formula: the pyrosulfate ion has the formula, S2O72- Apply rule #2 Assign known oxidation numbers: According to rule 2.7, the oxidation number of the O atoms is -2, so write this number above the O symbol in the formula. Because the oxidation number of the sulfur atoms is unknown, x is written above the S symbol. So now we have: Sx2O7-2)-2 Apply rule #3 Calculate remaining oxidation numbers, and verify the results: Multiplying the oxidation numbers by the subscripts learners can see that the S atoms contribute 2x and the O atoms contribute 7(-2) = -14 to the total oxidation number. To arrive at the correct charge, apply rule 3.2, 2x + (-14) = -2. Solving for x yields +6. Therefore, in S2O72- the oxidation number of the S atoms is +6 and the oxidation number of the O atoms is -2. The sum of the total oxidation numbers for each element is 2(+6) + 7(-2) = -2, which is the charge on the ion. Educators should give their learners a couple of practice problems like those below and observe their attempts to solve to verify that they have understood the process of assigning and determining oxidation numbers. PRACTICE SOLUTIONS Once learners have become utterly bored with assigning and determining oxidation numbers, you can reenergize them by showing videos of the chemistry of air bags! YouTube has a large number of videos regarding this reaction, one potential video is: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact=8&ved=0CCUQtwIwAWoVChMIwuW7jKOKxgIVTAiSCh3MDwAI&url=http%3A%2F%2Fwww.youtube.com%2Fwatch%3Fv%3Dvc4I4hvy_hM&ei=VeB6VYLnF8yQyATMn4BA&usg=AFQjCNGxvoRGbkr3otHX0ECEdB6x7vdShg&bvm=bv.95515949,d.aWw Tell the learners that redox reactions save lives!! Automobile airbags are inflated with nitrogen gas produced by two redox reactions. The gas generator in some airbags contains sodium azide (NaN3) and iron (III) oxide (rust). The mixture is automatically ignited during a head-on collision. When this occurs, sodium azide decomposes in a redox reaction to form sodium and nitrogen: 2NaN3 à2Na + 3N2(g) The sodium produced by this reaction is oxidized by iron (III) oxide: 6Na + Fe2O3 à3Na2O + 2Fe Activity 2 (45 minutes) HOW TO IDENTIFY REDOX REACTIONS AND WRITING HALF REACTIONS Show your learners a picture of the reaction of zinc metal with hydrochloric acid. Better yet, demonstrate this reaction in class. Inexpensive sources of zinc can be identified from the appendix. After demonstrating the reaction, direct learners to ask each other the essential question. Remind them that based upon our previous conversations they should be able to identify the main topic and create an appropriate question. Have learners post their questions to the parking lot. Review the posted questions and focus on the questions that ask: Is this a redox reaction? Congratulate and if possible, reward all the learners who were on the right track. Explain to them that hydrochloric acid is really just a solution of Cl– and H3O+ ions in water. The ‘acid’ component of HCL is the hydronium ion, H3O+, and the Cl– plays absolutely no role in the reaction pictured. So this reaction can be described as: 2 H3O+(aq) + Zn(s) à H2(g) + 2H2O(l) + Zn2+ (aq) Using the rules 2.1, 2.2, 2.6, 2.7, 3.1 and 3.2 from the SKILLS BOX above, we can assign oxidation numbers to all these atoms as follows: +1 -2 0 0 +1 -2 +2 2 H3O+(aq) + Zn(s) à H2(g) + 2H2O(l) + Zn2+ (aq) Upon examining the oxidation numbers, one can determine that the zinc atom changes from 0 to +2 and that two hydrogen atoms change from +1 to 0. So the answer to our question is yes! This is a redox reaction. Recall, in a redox reaction the oxidation numbers of atoms that are oxidized increase, and those of atoms that are reduced decrease. In the reaction pictured above, each zinc atom loses two electrons and is oxidized. Explain the your learners that one way to show only this half of the overall redox reaction is by writing a half-reaction for the change. Zn(s) à Zn2+(aq) + 2e– Notice when we write it this way we show electrons as products. Since oxidation and reduction always occur together, we can, similarly, show the half-reaction for reduction which show electrons as products like this: 2e– + 2H3O+(aq) àH2(g) + 2H2O(l) You finish describing half-reactions by telling your learners that if they add the two half-reactions together, they will get the overall redox reaction we began with: 2 H3O+(aq) + Zn(s) à H2(g) + 2H2O(l) + Zn2+ (aq) Key Terms: Half-reaction — the part of a reaction that involves only oxidation or reduction It is here that you may want to stop and remind your young charges of a couple of things. First, make sure your half-reactions contain enough electrons to keep the charges balanced. Secondly, they should understand that electrons aren’t just freely floating around in solution but are transferred directly from one substance or agent to the other. I’ve always taught my learners to remember oxidation and reduction using the following mnemonic: LEO the lion says “GER”: Loss of Electrons is Oxidation, Gain of Electrons is Reduction. Learners oftentimes complain that it doesn’t make sense to say that an agent which Gains electrons and hence gets larger is being reduced. Educators can take this opportunity to provide a cross-cutting competency history lesson regarding the development of scientific understandings. In this instance, the idea of reduction relates to the fact that when an ion or atoms gains electrons, its oxidation state is reduced to a lower value. The opposite process, oxidation, relates to the fact that elements lose electrons in the same way they do when combining with oxygen, taking on higher oxidation states. This terminology originated before Dymitri Mendeleev’s time, when the combining abilities of elements were studied by examining and comparing the proportions in their various oxides. From how these studies were carried out, to discussion about the principle scientists performing these experiments, educators can greatly expand the scope of this lesson to incorporate the multiple intelligences of their learners.[8] Substances that cause the oxidation of other substances are called oxidizing agents. They easily accept electrons and in doing so are reduced. Reducing agents cause reduction to happen and are themselves oxidized. Key Terms: Oxidizing agent – the substance that gains electrons in an oxidation-reduction reaction and is reduced Key Terms: Reducing agent — a substance that has the potential to reduce another substance. A good method for helping learners to become more interested in the processes of oxidation and reduction is to assign them to work in small cooperative groups whose task it is to research three naturally occurring redox reactions. Direct the learners to share their discoveries with the class by writing the equations for these reactions (the reactions they researched) using electronic presentation media such as Apple’s Keynote or Microsoft’s PowerPoint. They should illustrate their finds with images and present them on a day when a change of pace is needed. Day 2 Electrode potentials and the fruit cell “Suppose we make two deep slits several centimeters apart in a lemon and place a strip of zinc in one slit and a strip of copper in the other. The strip of zinc and lemon solution in which it is immersed constitute the one half-cell, while the strip of copper and lemon solution in which it is immersed constitute the other half-cell. Membranes in the lemon keep the two half-cells separate, while the citric acid of the lemon allows for current to flow between the electrodes. If the two metal strips are connected with an external copper wire, electrons flow through the wire from the zinc (the reducing agent) to copper (the oxidizing agent). The combination is an electrochemical cell (also known as a galvanic or voltaic cell), and the metal strips at which the transfer of electrons occur are called electrodes. The zinc electrode at which oxidation occurs (electrons are lost) is called the anode, while the copper electrode at which reduction occurs (electrons are gained) is called the cathode. The potential difference of a galvanic cell (expressed in volts) is a measure of how readily electrons flow from one electrode to the other, and can be thought of as the force which pushes the electrons through the wire. Do different combinations of metals produce voltages? We’ll perform the following experiments to answer this question. Obtain similar sized strips of a variety of metals, such as copper, iron, magnesium, tin, lead, nickel, aluminum, carbon, and zinc. Appendix A indicates common sources for numerous materials used in this and other activities, but it should be realized that these may not be as pure as those acquired from a chemical supply house. Rub the metal strips with steel wool to remove oil and oxide coats. Make two slits in the lemon (any citrus fruit will suffice) a few centimeters apart (far apart so that the two strips of metal do not touch when inserted in the lemon). Place a piece of copper in one slit and a piece of iron in the other slit. Connect the leads of a voltmeter (multimeter) to both strips as shown below. Reverse the leads if necessary to obtain a positive voltage reading. Record the voltage in the following table: Voltages for Cells with Copper Cathodes FRUIT FRUIT Leave the copper strip in the lemon. Remove the iron strip and test the potential difference between copper and each of the following: magnesium, tin, nickel, aluminum, zinc. Use the voltages to rank the metals in terms of ease with which electrons are lost to copper. The more easily electrons are transferred to copper, the higher the voltage of the galvanic cell. Repeat the procedure using tomatoes, oranges, potatoes or apples for the wet cell. Does the type of fruit used for wet cell affect the ranking of potential differences? Do you think silver loses electrons more easily than copper? How could you find out? Questions Batteries Concepts to Investigate: Batteries, oxidation-reduction, electrochemical cells (voltaic cells, galvanic cells), potential, parallel circuits, series circuits, instrumentation, current detection, magnetism. Materials: Part 1: Lemons or other citrus fruit, copper and zinc sheeting or to other pair of metals with very different reduction potentials (see Appendix B), galvanometer, multimeter, steel wool; Part 2U: Salt, compass, fine insulated copper wire, absorbent paper towel or filter paper, steel wool, and sheets of iron and copper, or U.S. copper pennies (pre-1982)[10] and silver dimes, quarters or half-dollars (pre-1964).[11] Safety: Do not eat food used in this or any other experiment. Discard the fruits after completion of the activity. Principles and Procedures: In the early years of the automobile it was necessary to turn the crank of a magneto (an alternator with permanent magnets) to generate the current necessary to start the automobile engine. In response to consumer demand for ran easier system, the automobile industry introduced the lead-acid storage battery, a form of which is still in use today. In a car battery, lead serves as the anode, and lead oxide as the cathode. Both electrodes are immersed in a solution of sulfuric acid, which serves as the electrolyte. The balanced redox reaction is: Pb(s) + PbO2(s) + 2H+(aq) + 2HSO–(aq) -à 2PbSO4(s) + 2H2O(l) Eocell = 2V Note that the potential of this reaction is merely 2 volts, too low to be of use in starting an automobile. To remedy this situation, developers paced six lead-acid cells in series to produce a 12-volt battery. A battery is voltaic cell or series of cells. When cells are connected in series, a battery with higher voltage is produced. Can you create a battery from the fruit cell wet-cells developed in the previous activity? Part 1: Batteries; wet cells in series: Prepare three fruit wet-cells using copper as the cathode and zinc (or other reactive metal) as the anode as shown below. Place two cells in series by connecting the cathode of one to the anode of the next. Record this voltage and then connect additional cells in series and record these voltages in table 1. Table 1 – Series vs. Parallel Circuits Have learners again attempt to arrive at their own questions regarding this experiment. Encourage them to base their questions upon their observations which have been reduced to data. Refer them to their data table. Have learners post their questions to the parking lot. Examine the posted questions and select one which asks — Is voltage additive when cells are added in series? With enough cells you should be able to produce a 12-volt battery, but don’t expect it to start your car! Although your battery may have sufficient voltage, it does not generate sufficient amperage (current) to start a car, or much else for that matter. The current a battery generates is inversely related to the internal resistance (which for your cells is quite high). The resistance is reduced and the current increase by improving the electrolyte solution and by increasing the surface area of the electrodes. The greater the surface area, the greater the capacity for oxidation and reduction, and the greater the number of electrons transferred. The electrodes in automobiles batteries are made of layered grills, providing a large surface area on which oxidation and reduction may take place. What is the advantage of placing batteries in parallel as shown in the next figure? Using a multimeter and galvanometer, measure the resistance and current across parallel and series cells as indicated in table 1. What effect does arrangement have upon resistance and current? Part 2: Voltaic pile: In 1800, Italian physicist Alessandro Volta introduced the world’s first operational battery. Volta’s battery consisted of a stack of alternating zinc and silver disks separated by layers of cloth soaked in a solution of either sodium hydroxide or brine. By combining zinc/silver cells in series, Volta produced a battery with appreciable voltage and power. It is possible to make a simple “voltaic pile” battery using any two metals with significantly different potentials. Construct a simple cell by inserting a piece of saltwater soaked paper towel between sheets of polished copper (use steel wool to polish the copper or a pre-1982 penny will suffice) and iron (an iron washer will suffice) as shown below. This image is actually several voltaic cells stacked together. You should construct at least three of them and arrange in series. Measure the voltage using a multimeter. Now connect a second and third cell in series as shown in the figure below. Regarding this image, each penny should be considered single cell and connected in series. (refer to the lemon series circuit shown earlier if confused). Is the voltage additive? A battery is a set of cells connected is series. A 12-volt car battery is composed of six lead/lead oxide cells, each with a voltage of approximately 2 volts. On the basis of your voltage measurements, how many of your home-made copper/iron cells would you need to generate 12 volts? Try it! Although you may be able to generate high voltage with your copper/iron battery, you will notice that it has insufficient power to start a car or even light a small light bulb. The voltage of a battery is determined by the chemical energy available when an electron is transferred from one electrode to the other, and thus is a function of the types of electrodes, not their size. However, the current from a cell or battery is a function of the resistance of the total circuit, including the battery itself. While the car battery has a very low internal resistance and is able to deliver strong current, your simple battery has a very high internal resistance and is therefore unable to deliver as much current. The resistance of a cell can be decreased by increasing the surface area of the electrodes. Use large sheets of copper and iron or other metal and compare the amperage of batteries with these larger electrodes with those that have small electrodes. When Volta constructed his battery (voltaic pile), he did so by stacking two different metals in series, each separated from the others by a felt pad soaked with an electrolyte. You can make a voltaic pile, and eliminate the need for patch cables, by stacking cells as shown below. Make wads of filter paper or paper towels by folding them back and forth on each other four or five times. Soak the wads in salt solution, and then gently squeeze excess salt solution out of the wads before placing them between alternating metals as shown. You can also make a voltaic pile out of coins by alternating copper (pre-1982 U.S. pennies) and silver coins (pre-1964 U.S. dimes, quarters, or half-dollars). Whenever making a voltaic pile, make certain that the coins do not touch each other, that the towel wads do not touch, and that the electrolyte solution (saltwater) does not run between one wad and another. Avoid using iron washers because towels wads may touch in the center of the washer. The energy content of a voltaic cell is determined by the quantity of electrons that can be transferred from the anode to the cathode. When the electrodes have been consumed, the voltage of the cell falls to zero and will not recover. Since the current produced by your copper/zinc battery is small, it will maintain its potential for a significant amount of time.”[18] Lithium Ion Batteries Your learners will need your assistance connecting the chemistry previously taught to the lithium-ion battery which powers their phone. You have previously explained how a battery works, that is a device that stores electricity and delivers that electrical energy through a controlled route. Review with your learners that a battery is composed of a series of cells. Refer them back to the earlier experiments where they themselves constructed battery cells. Review with the learners that each cell of a battery has these essential components: an anode, a cathode, and the electrolyte. Connecting the anode and cathode together through an electrical connector like a copper wire allows electrons to flow through from the anode through the wire to the cathode creating an electrical current. Likewise, the electrolyte conducts positive current in the from of positive ions called cations. Point out to the learners that the materials used for each of these components determine a batteries characteristic which includes its capacity. Capacity is defined as the total amount of energy—and its voltage. Voltage can be defined as the amount of energy per electron. Use the following analogy to help the learners understand the operation of a battery. Tell them to imagine that a battery is similar to a tank of water which is being drained by a hose. The volume of the tank can be considered to be the capacity of the battery, and the pressure which is built up in the hose is its voltage. The materials which composed the anode and cathode terminals of the lithium ion battery have been selected for very specific reasons. It is not sufficient that the anode terminal is the electron donor, nor that the cathode terminal be able to receive them. Now would be an excellent time to introduce learners to a discussion of the types of materials chosen for the cathode. Modern day cathode materials are lithium-metal oxides. Some examples of these oxides are LiCoO2 , LiMn2O4 and LFePO4. Current anode materials are lithium-alloying materials. There exists an opportunity to expand this unit by assigning independent research on alloys. Help learners to make the connection that different batteries use different materials for specific reasons but since nearly all the cathodes used in cell phones require the use of lithium the batteries are generically called lithium ion batteries. The difference between the electrode potentials of the cathode and anode determine the voltage of the battery cell. Recall that these two terminals are separated by an electrolyte which is either a liquid or a gel. Safe battery operation requires electrolytes with a long shelf life. Tell learners that shelf-life refers to how long the battery component may be successfully used before being discarded. In the lithium-ion battery, the lithium ion is the cation that travels from anode to cathode. Ask the learners why they think lithium is most frequently used as opposed to another group 1 alkali metal. Lithium (Li) is used because it is the lightest element capable of forming the +1 ion. It is ionized to form Li+ plus one electron. Li à Li+ + e_ The electrolyte is usually a combination of lithium salts such as LiClO4. This combination of materials results in an overall voltage of 3.6 Volts (V). Remind learners that the unit of voltage is named after Alessandro Volta who invented the voltaic pile. Smile now, because you’ve covered this intensively earlier in this unit. Make connections with learner’s previous knowledge by explaining the significance of the 3.6V produced by the use of the lithium ion battery. Tell learners that 3.6V is more than twice that of an AA alkaline battery, meaning that lithium ion batteries have a better energy per volume ratio called energy density. The energy density of lithium ion batteries is better than standard alkaline batteries and even other rechargeable battery types such as nickel-metal hydrides. This is directly related to lithium being the lightest group 1 metal. What this really means is that the lithium ion can carry a positive charge in a very small amount of space. Have learners reflect on this fact, an electron is always the same size but the positive ion which results from the loss of that electron varies with the size of the parent atom. Tell the learners to look at the periodic table and confirm for themselves that lithium is the smallest metal capable of forming a positive ion. It is often helpful to give learners a competing perspective and this is especially useful when discussing energy density. Lithium ion batteries store much, much less energy than other substances such as gasoline. Electric cars, like our cell phones, typically use lithium ion batteries as well. The table below is a comparison of the energy densities of various fuels including the lithium ion battery which powers the modern electric car. Additional expansions of this curriculum would be to study the limitation of Tesla’s electric car due to limitations with the energy density of the battery. As you can see from the above chart, increasing the energy density of the lithium ion battery is a necessity before electric vehicles become more competitive with vehicles powered by traditional means. This is much less an issue in respect to cell phones since all such phones are powered by batteries and lithium is king because of size and energy density. Lithium-ion batteries and all rechargeable batteries are recharged by reversing the anode and cathode reactions in through the use of electric current sourced from wall outlets. When the cell phone is plugged into a charger, the cathode becomes the anode and vice-versa. The final topic to be discussed in this unit involves a consideration of the social and economic costs of the use of modern day cell phones. Learners should be made to consider the populations of persons world-wide who own cell phones in comparison to the populations of persons who work in industries where the basic components of the cell phone are sourced. The majority of the information which follows was acquired during the TIP seminar titled Energy, Sustainability, and Environment – 2015. So, educators will ask the learners ‘what does a cell phone cost?’ Many learners will respond “$300 bucks,” ‘mine’s was free” and so forth. Chuckle and prod them to consider the question a little more deeply while displaying some of the pictures below: Many of the minerals used in the cell phone components are sourced from countries with poor economies. One such material is coltan, a mineral used in the manufacture of capacitors. Coltan is short for Columbite-tantalite – a black tar-like mineral found in major quantities in the Congo. [21]Capacitors are electric components capable of storing electric energy.[22] While this sounds a lot like the battery we’ve been discussing it is not. Challenge your learners to create a Venn diagram using the words BATTERY and CAPACITOR. The human labor that is often used in these regions to mine minerals from the Earth is children. The lack of responsible governments and/or agencies in these areas leads to difficult and dangerous work environments that dramatically shorten the life spans of these young people. The money that is procured from the sale of the mined minerals is often used to fund genocidal wars. Rwanda, Uganda, Burundi and their proxy militias are the primary exploiters of coltan in the Congo. In an 18 month period Rwanda made $250 million as a result of exploitation of coltan in the Congo. Although Rwanda and Uganda possess little or no coltan, during the period of the war in the Congo, their exports escalated exponentially. For example, Rwanda’s coltan export went from less than 50 tons in 1995 to almost 250 tons in 1998. Zero cassiterite was transported from the Congo to Uganda in 1998, however by 2000 151 drums were transported. The United Nations notes in its 2001 report on the Illegal Exploitation of Natural Resources in the Congo that “The consequences of illegal exploitation has been twofold: (a) massive availability of financial resources for the Rwandan Patriotic Army, and the individual enrichment of top Ugandan military commanders and civilians; (b) the emergence of illegal networks headed by either top military officers or businessmen.”[23] This unit is concluded with engaging learners in conversations about where the unused and discarded cell phones and related components are sent once we ‘upgrade.’ This is a valuable opportunity to encourage learners to seek out those businesses who have pledged to recycle used cell phones ethically. You will find that these and other pictures are quite riveting and many learners will want to do something to help those less fortunate. I encourage you to consider service learning projects to capitalize on the riveted audience I’ve just delivered to you! [1] http://www.physicsclassroom.com/class/circuits/Lesson-4/Series-Circuits [2] http://www.mts.net/~alou/Chemistry%2011/images/lesson%202j.jpg [3] http://www.chem.uiuc.edu/clcwebsite/elec.html [4] http://www.physicsclassroom.com/class/energy/u5l1b.cfm [5] Herr, Norman, and James B. Cunningham. Hands-on Chemistry Activities with Real-life Applications. West Nyack, NY: Center for Applied Research in Education, 1999. Print. [6] Herr, Norman, and James B. Cunningham. Hands-on Chemistry Activities with Real-life Applications. West Nyack, NY: Center for Applied Research in Education, 1999. Print. [7] http://image.slidesharecdn.com/replacementreations-101004145446-phpapp01/95/replacement-reactions-5-728.jpg?cb=1286218451 [8] Myers, R. Thomas., Keith B. Oldham, and Salvatore Tocci. Holt Chemistry. Orlando: Holt, Rinehart and Winston, 2006. Print. [9] http://www.thenakedscientists.com/HTML/uploads/RTEmagicC_LemonBattery-diagram_01.png.png [10] http://en.wikipedia.org/wiki/Penny_%28United_States_coin%29 [11] http://en.wikipedia.org/wiki/Kennedy_half_dollar [12] http://paksc.org/pk/images/stories/images/fruit%20cell%203.JPG [13] http://upload.wikimedia.org/wikipedia/commons/thumb/0/05/Lemon_Battery_With_LED.svg/220px-Lemon_Battery_With_LED.svg.png [14] http://www.eveready.co.za/images/cmsimages/big/page_419_490_Voltaic%20pile.jpg [15] http://cdn.instructables.com/F1F/4JY7/HBFFJJ4B/F1F4JY7HBFFJJ4B.LARGE.jpg [16] https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcQ6KwCuRUw9qMoZeGzou0YUCVqev32CBusyOUSrYz0dzNE9gX0K [17] https://www.codehosting.net/blog/images/vpile1.jpg [18] Herr, Norman, and James B. Cunningham. Hands-on Chemistry Activities with Real-life Applications. West Nyack, NY: Center for Applied Research in Education, 1999. Print [19] http://www.exxonmobilperspectives.com/wp-content/uploads/2011/12/Energy-Density-Comparison.png [20] http://www.sciencemag.org/content/296/5571/1224/F2.medium.gif [21] http://www.friendsofthecongo.org/resource-center/coltan.html [22] http://www.electronics-tutorials.ws/capacitor/cap_1.html [23] http://www.friendsofthecongo.org/resource-center/coltan.html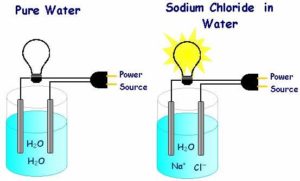
Anode
Anode
Iron
V
V
Tin
V
V
Zinc
V
V
Nickel
V
V
magnesium
V
V
aluminum
V
V
Potential Volts, V
Current Milliamps. mA
Resistance Ohms, Ω
Single cell
2 cells in series
3 cells in series
2 cells in parallel
3 cells in parallel



For Educators http://www.historyworld.net/wrldhis/PlainTextHistories.asp?historyid=aa93 :article about first cell phone http://www.archives.gov/education/lessons/telephone-light-patents/ : article about first telephone http://www.theatlantic.com/national/archive/2012/05/do-cell-phones-belong-in-the-classroom/257325/ : paper which discusses use of cell phones in classrooms https://www.commonsensemedia.org/educators/blog/for-stem-learning-real-world-application-matters : helps make connections science to the real-world http://www.pdesas.org/standard/StandardsBrowser/76626 : science standards http://www.pdesas.org/standard/StandardsBrowser/162528 : science standards http://www.nextgenscience.org/hsps1-matter-interactions : science standards http://www.physicsclassroom.com/class/circuits/Lesson-4/Series-Circuits : discusses series circuits http://www.mts.net/~alou/Chemistry%2011/images/lesson%202j.jpg : image http://www.chem.uiuc.edu/clcwebsite/elec.html : provides further explanations regarding electric circuits http://www.physicsclassroom.com/class/energy/u5l1b.cfm : provides background on potential energy : tutorial on replacement reactions http://www.thenakedscientists.com/HTML/uploads/RTEmagicC_LemonBattery-diagram_01.png.png : image Myers, R. Thomas., Keith B. Oldham, and Salvatore Tocci. Holt Chemistry. Orlando: Holt, Rinehart and Winston, 2006. Print. : primary chemistry reference document http://en.wikipedia.org/wiki/Penny_%28United_States_coin%29 : history of copper pennies http://en.wikipedia.org/wiki/Kennedy_half_dollar : history of silver dollars http://paksc.org/pk/images/stories/images/fruit%20cell%203.JPG : image http://www.eveready.co.za/images/cmsimages/big/page_419_490_Voltaic%20pile.jpg http://cdn.instructables.com/F1F/4JY7/HBFFJJ4B/F1F4JY7HBFFJJ4B.LARGE.jpg : image https://www.codehosting.net/blog/images/vpile1.jpg : image Herr, Norman, and James B. Cunningham. Hands-on Chemistry Activities with Real-life Applications. West Nyack, NY: Center for Applied Research in Education, 1999. Print :print book where I acquired most of the lab experiments http://www.exxonmobilperspectives.com/wp-content/uploads/2011/12/Energy-Density-Comparison.png : provides useful comparisons of the energy densities of different materials http://www.sciencemag.org/content/296/5571/1224/F2.medium.gif : image http://www.friendsofthecongo.org/resource-center/coltan.html : discusses issues faced by the Congo http://www.electronics-tutorials.ws/capacitor/cap_1.html : Tutorial on capacitors Annotated reading list for learners http://www.friendsofthecongo.org/resource-center/coltan.html : discusses the issues faced by the Congo
Annotated list of materials for classroom use (Referred to as Appendix A in curriculum unit) : Cheap sources of chemicals for use in laboratory experiments Appendix B Table of standard reduction potentials
Chemical
Formula
Source/Description
Sucrose
C12H22O11
Table sugar is available at grocery stores
Sulfur
S
Flowers of sulfur is sold at some garden stores
Sulfuric acid
H
Battery acid, also known as oil of vitriol, is sulfuric acid and may be obtained at some auto supply stores
Zinc
Zn
Recent US pennies (1982-present) are 97.5% zinc with 2.5% copper coating.
Sodium hydroxide
NaOH
Known also as caustic soda and lye, sodium hydroxide is used in many commercial drain cleaners
Reaction
Eo
Zn2+ + 2e– —> Zn
-0.76
Cu2+ + 2e– —> Cu
+0.34
Sn4+ + 2e– —> Sn2+
+0.15
PbO2 + 4H+ + SO42- + 2e– —> PbSO4(s) + 2H2O
+1.69
PbO2 + 4H+ + 2e– —> Pb2+ + 2H2O
+1.46